
Chemistry, 02.12.2019 23:31 genyjoannerubiera
Which of the following reactions results in a decrease in entropy? 2so2 (g) + o2 (g) yields 2so3 (g) 2nh3 (g) yields n2 (g) + 3h2 (g) 2h2o2 (aq) yields 2h2o (l) + o2 (g) nahco3 (aq) + hcl (aq) yields nacl (aq) + h2o (l) + co2 (g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2


Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

You know the right answer?
Which of the following reactions results in a decrease in entropy? 2so2 (g) + o2 (g) yields 2so3 (g...
Questions

Mathematics, 09.12.2021 03:10

Advanced Placement (AP), 09.12.2021 03:10

Mathematics, 09.12.2021 03:10

History, 09.12.2021 03:10


History, 09.12.2021 03:10

Engineering, 09.12.2021 03:10

Mathematics, 09.12.2021 03:10

Mathematics, 09.12.2021 03:10

SAT, 09.12.2021 03:10


Mathematics, 09.12.2021 03:10

Computers and Technology, 09.12.2021 03:10

History, 09.12.2021 03:10


Computers and Technology, 09.12.2021 03:10




Biology, 09.12.2021 03:10

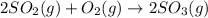 results in a decrease in entropy as 3 moles is giving 2 moles.
results in a decrease in entropy as 3 moles is giving 2 moles.

