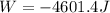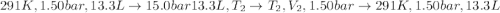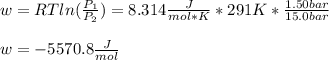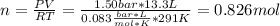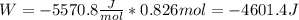An ideal gas described by Ti=291K, Pi=1.50bar, and Vi=13.3L is heated at constant volume until P=15.0bar. It then undergoes a reversible isothermal expansion until P=1.50bar. It is then restored to its original state by the extraction of heat at constant pressure. Calculate w for step 2 (P, Vi, T → Pi, V2, T).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
An ideal gas described by Ti=291K, Pi=1.50bar, and Vi=13.3L is heated at constant volume until P=15....
Questions

English, 24.02.2021 23:40


Spanish, 24.02.2021 23:40

Mathematics, 24.02.2021 23:40


Arts, 24.02.2021 23:40

Mathematics, 24.02.2021 23:40

Mathematics, 24.02.2021 23:40

Geography, 24.02.2021 23:40


Mathematics, 24.02.2021 23:40


Mathematics, 24.02.2021 23:40

Mathematics, 24.02.2021 23:40

Mathematics, 24.02.2021 23:40


Mathematics, 24.02.2021 23:40

English, 24.02.2021 23:40

History, 24.02.2021 23:40

Biology, 24.02.2021 23:40