
Chemistry, 23.03.2020 16:58 mistiehaas
A 850.0 mL cylinder containing B2H6 at a pressure of 0.900 atm is connected by a valve to 1000 mL cylinder containing C6H6 at 608.0 torr pressure. Calculate the partial pressure (torr) of C6H6 when the valve is opened.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1

Chemistry, 23.06.2019 19:40
Aresearcher finds that white bread contains more preservatives and has a higher moisture content than wheat bread. she designs a test to sermons whether the type of bread, white or wheat, affects the amount of mold growth. what must be done for the researcher to accurately measure the dependent variable?
Answers: 1

Chemistry, 23.06.2019 22:30
Write the formula for the conjugate base acid of hexanoic acid
Answers: 1
You know the right answer?
A 850.0 mL cylinder containing B2H6 at a pressure of 0.900 atm is connected by a valve to 1000 mL cy...
Questions



Biology, 07.10.2020 23:01

Law, 07.10.2020 23:01


Business, 07.10.2020 23:01

Mathematics, 07.10.2020 23:01




English, 07.10.2020 23:01

Physics, 07.10.2020 23:01


French, 07.10.2020 23:01

Engineering, 07.10.2020 23:01

History, 07.10.2020 23:01



History, 07.10.2020 23:01

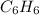 when the valve is opened is 328.6 Torr.
when the valve is opened is 328.6 Torr.

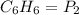

 ( Boyle's law)
( Boyle's law)


