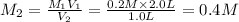Read the statement.
Some amount of water is evaporated from a 2.0 L, 0.2 M NaI solution,...

Chemistry, 23.03.2020 04:29 Poohpooh6667
Read the statement.
Some amount of water is evaporated from a 2.0 L, 0.2 M NaI solution, to from a 1.0 L solution. The molar mass of NaI is 150 g/mol.
What is the final concentration of NaI solution in?
30 g/L
15 g/L
60 g/L
45 g/L

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

You know the right answer?
Questions

Mathematics, 09.02.2021 14:00

English, 09.02.2021 14:00


History, 09.02.2021 14:00

Mathematics, 09.02.2021 14:00






Mathematics, 09.02.2021 14:00

Mathematics, 09.02.2021 14:00

Social Studies, 09.02.2021 14:00



Mathematics, 09.02.2021 14:00


History, 09.02.2021 14:00


Mathematics, 09.02.2021 14:00

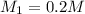
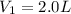
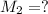
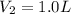
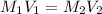 ( dilution)
( dilution)