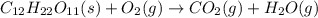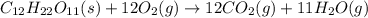Chemistry, 22.03.2020 06:18 jordynj6363
Predict the products for the reaction of burning sucrose, C12H22O11(s), with excess oxygen, O2(g)
(A) 6CO(g), 6CO2(g), 11H2O(g
B)12CO(g), 11H2O(g)
C)12CO2(g), 11H2O(g)
D)24CO2(g), 22H2O(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Predict the products for the reaction of burning sucrose, C12H22O11(s), with excess oxygen, O2(g)
Questions







History, 04.12.2020 04:30



Spanish, 04.12.2020 04:30

Biology, 04.12.2020 04:30


Social Studies, 04.12.2020 04:30


Mathematics, 04.12.2020 04:30

Mathematics, 04.12.2020 04:30



Chemistry, 04.12.2020 04:30

Arts, 04.12.2020 04:30