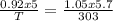A sample of argon has a volume of 5.0 dm^3 and the pressure is 0.92 atm.
If the final temperat...

Chemistry, 22.03.2020 02:39 dragon2998
A sample of argon has a volume of 5.0 dm^3 and the pressure is 0.92 atm.
If the final temperature is 30.° C, the final volume is 5.7 L, and the final
pressure is 800. mm Hg, what was the initial temperature of the argon?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Questions

Mathematics, 01.10.2019 06:10

History, 01.10.2019 06:10

Social Studies, 01.10.2019 06:10


History, 01.10.2019 06:10

Mathematics, 01.10.2019 06:10


Mathematics, 01.10.2019 06:10


History, 01.10.2019 06:10

Mathematics, 01.10.2019 06:10

Chemistry, 01.10.2019 06:10


Mathematics, 01.10.2019 06:10


Mathematics, 01.10.2019 06:10

Mathematics, 01.10.2019 06:10

Mathematics, 01.10.2019 06:10


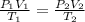
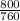 = 1.05atm
= 1.05atm