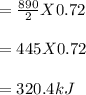Chemistry, 21.03.2020 22:19 annaebrown9737
How many kilojoules of heat are released when 0.72 mole of oxygen gas are used to combust methane?
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (ℓ) + 890kJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2

Chemistry, 23.06.2019 09:30
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2
You know the right answer?
How many kilojoules of heat are released when 0.72 mole of oxygen gas are used to combust methane?
Questions

Geography, 07.09.2021 08:30

English, 07.09.2021 08:30

Mathematics, 07.09.2021 08:30


History, 07.09.2021 08:30



Mathematics, 07.09.2021 08:30

History, 07.09.2021 08:30

Mathematics, 07.09.2021 08:40

Mathematics, 07.09.2021 08:40



Mathematics, 07.09.2021 08:40


Chemistry, 07.09.2021 08:40

Mathematics, 07.09.2021 08:50

Social Studies, 07.09.2021 08:50