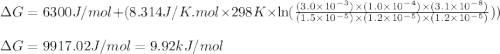Given the following: [G3P] = 1.5x10-5M; [BPG] = 3.0x10-3M ; [NAD+] = 1.2x10-5M; [NADH]=1.0x10-4 ; [HPO42-]= 1.2x10-5 M; pH = 7.5 ; DGo=6.3 kJ/mol
Glyceraldehyde3-phosphate + NAD+ + HPO42- ---> 1,3-Biphosphoglycerate + NADH + H+
Predict whether this reaction will be spontaneous.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Given the following: [G3P] = 1.5x10-5M; [BPG] = 3.0x10-3M ; [NAD+] = 1.2x10-5M; [NADH]=1.0x10-4 ; [H...
Questions

Physics, 08.01.2021 05:30

Biology, 08.01.2021 05:30

Chemistry, 08.01.2021 05:30





Physics, 08.01.2021 05:30

Mathematics, 08.01.2021 05:30

Mathematics, 08.01.2021 05:30




Geography, 08.01.2021 05:30





Mathematics, 08.01.2021 05:40

English, 08.01.2021 05:40

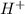 concentration, we use the equation:
concentration, we use the equation:![pH=-\log[H^+]](/tpl/images/0557/5671/cf945.png)
![7.5=-\log [H^+]](/tpl/images/0557/5671/95063.png)
![[H^+]=10^{-7.5)=3.1\times 10^{-8}M](/tpl/images/0557/5671/67b53.png)
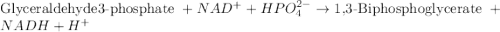
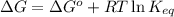
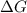 = free energy of the reaction
= free energy of the reaction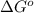 = standard Gibbs free energy = 6.3 kJ/mol = 6300 J/mol (Conversion factor: 1kJ = 1000J)
= standard Gibbs free energy = 6.3 kJ/mol = 6300 J/mol (Conversion factor: 1kJ = 1000J)![25^oC=[273+25]K=298K](/tpl/images/0557/5671/0e82f.png)
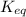 = Ratio of concentration of products and reactants =
= Ratio of concentration of products and reactants = ![\frac{[BPG][NaDH][H^+]}{[G_3P][NAD^+][HPO_4^{2-}]}](/tpl/images/0557/5671/dd6ba.png)
![[BPG]=3.0\times 10^{-3}M](/tpl/images/0557/5671/38e24.png)
![[NADH]=1.0\times 10^{-4}M](/tpl/images/0557/5671/94c0b.png)
![[H^+]=3.1\times 10^{-8}M](/tpl/images/0557/5671/2f9b6.png)
![[G_3P]=1.5\times 10^{-5}M](/tpl/images/0557/5671/aafff.png)
![[NAD^+]=1.2\times 10^{-5}M](/tpl/images/0557/5671/711eb.png)
![[HPO_4^{2-}]=1.2\times 10^{-5}M](/tpl/images/0557/5671/2ab39.png)