Chemistry, 21.03.2020 08:35 alexusjones6042
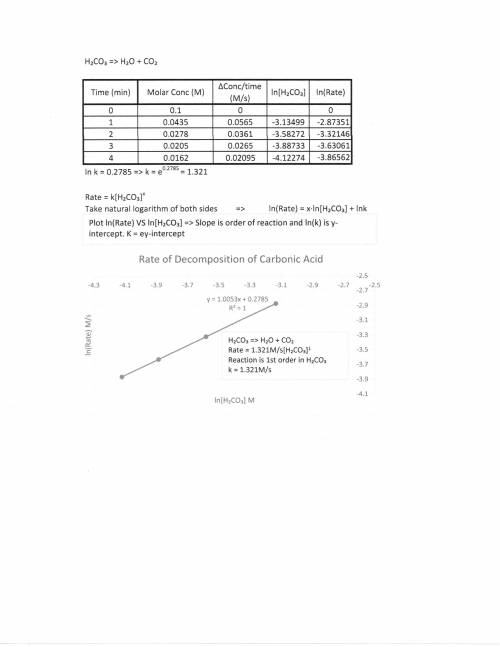
A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) →H2O(aq)+CO2(aq)
He fills a reaction vessel with H2CO3 and measures its concentration as the reaction proceeds:
time (minutes) H2CO3
0 0.100M
1.0 0.0435M
2.0 0.0278M
3.0 0.0205M
4.0 0.0162M
write the reate law for this reaction: k ?
calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbols.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) →H2O(aq)+CO2(aq)
Questions

Biology, 30.06.2019 01:30







Arts, 30.06.2019 01:30