
Chemistry, 21.03.2020 04:52 jackieanguiano3700
He following mechanism has been proposed for the gas-phase reaction of chloroform (CHCl3) and chlorine. Cl2 ⇌ 2Cl (fast, reversible) Cl + CHCl3 → HCl + CCl3 (slow) Cl + CCl3 → CCl4 (fast) What rate law does this mechanism predict? (Choose from the list below and enter your answers in alphabetical order, e. g. ABC ). A)k G) [CCl3]1/2 M) [HCl]2 B) [Cl] H) [HCl]1/2 N) [Cl2]2 C) [CHCl3] I) [Cl2]1/2 O) [Cl]2 D) [CCl3] J) [Cl]1/2 P) [CHCl3]2 E) [HCl] K) [CHCl3]1/2 F) [Cl2] L) [CCl3]2 Tries 0/99 Decide which of the following reactive intermediates could exist for the reaction above. Cl Cl2 CHCl3 CCl3 CCl4 Tries 0/99

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Why do you suppose the structural polysaccharide cellulose does not contain branches? why do you suppose the structural polysaccharide cellulose does not contain branches? branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby decreasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby increasing the rigidity and strength of the microfibrils. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby increasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby decreasing the rigidity and strength of the microfibrils.
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
He following mechanism has been proposed for the gas-phase reaction of chloroform (CHCl3) and chlori...
Questions

Mathematics, 17.04.2020 16:14

Mathematics, 17.04.2020 16:14

Mathematics, 17.04.2020 16:14


Spanish, 17.04.2020 16:14




Physics, 17.04.2020 16:14

Computers and Technology, 17.04.2020 16:14

Mathematics, 17.04.2020 16:14


Computers and Technology, 17.04.2020 16:15

Mathematics, 17.04.2020 16:15



Mathematics, 17.04.2020 16:15

Business, 17.04.2020 16:15


Mathematics, 17.04.2020 16:15

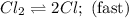
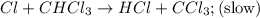
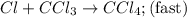
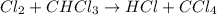
![\text{Rate}=K_2[Cl][CHCl_3]](/tpl/images/0557/3211/4079f.png) ......(1)
......(1)![K_1=\frac{[Cl]^2}{[Cl_2]}](/tpl/images/0557/3211/5c3f3.png)
![[Cl]=\sqrt{K_1[Cl_2]}](/tpl/images/0557/3211/af496.png)
![K_3=\frac{[CCl_4]}{[CHCl_3][Cl]}](/tpl/images/0557/3211/3404e.png)
![[Cl]=\frac{[CCl_4]}{K_3\times [CCl_3]}](/tpl/images/0557/3211/7b25a.png)
![\text{Rate}=K_2(\sqrt{K_1[Cl_2]}\times (\frac{[CCl_4]}{K_3[CCl_3]})[CHCl_3]\\\\\text{Rate}=k[Cl]^{1/2}[CCl_4][CCl_3]^{-1}[CHCl_3]](/tpl/images/0557/3211/b7240.png)


