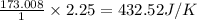Chemistry, 21.03.2020 04:26 zitterkoph
Consider the reaction 2CO(g) + O2(g)2CO2(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.25 moles of CO(g) react at standard conditions. S°surroundings = J/K

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
Consider the reaction 2CO(g) + O2(g)2CO2(g) Using standard thermodynamic data at 298K, calculate the...
Questions

Mathematics, 26.03.2021 03:00

Mathematics, 26.03.2021 03:00


English, 26.03.2021 03:00


English, 26.03.2021 03:00



Mathematics, 26.03.2021 03:00


Mathematics, 26.03.2021 03:00



Mathematics, 26.03.2021 03:00


Mathematics, 26.03.2021 03:00

Mathematics, 26.03.2021 03:00



Mathematics, 26.03.2021 03:00

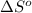 for the surrounding when given amount of CO is reacted is 432.52 J/K
for the surrounding when given amount of CO is reacted is 432.52 J/K![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0557/2961/52737.png)
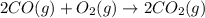
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(CO_2(g))})]-[(1\times \Delta S^o_{(O_2(g))})+(2\times \Delta S^o_{(CO(g))})]](/tpl/images/0557/2961/30479.png)
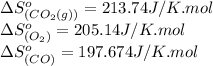
![\Delta S^o_{rxn}=[(2\times (213.74))]-[(1\times (205.14))+(2\times (197.674))]\\\\\Delta S^o_{rxn}=-173.008J/K](/tpl/images/0557/2961/a0064.png)