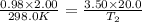Chemistry, 21.03.2020 03:15 gshreya2005
2.00 liters of hydrogen, originally at 25.0 °C and 750.0 mm of mercury, are heated until a volume of 20.0 liters and a pressure of 3.50 atmospheres is reached. What is the new temperature?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3


Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
2.00 liters of hydrogen, originally at 25.0 °C and 750.0 mm of mercury, are heated until a volume of...
Questions

English, 03.03.2021 18:10

History, 03.03.2021 18:10

Mathematics, 03.03.2021 18:10

History, 03.03.2021 18:10


Mathematics, 03.03.2021 18:10


History, 03.03.2021 18:10

Physics, 03.03.2021 18:10


Mathematics, 03.03.2021 18:10


Mathematics, 03.03.2021 18:10

Mathematics, 03.03.2021 18:10


Chemistry, 03.03.2021 18:10

Mathematics, 03.03.2021 18:10

Mathematics, 03.03.2021 18:10


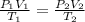
 = initial pressure of gas = 750.0 mm Hg = 0.98 atm (760mmHg=1atm)
= initial pressure of gas = 750.0 mm Hg = 0.98 atm (760mmHg=1atm) = final pressure of gas = 3.50 atm
= final pressure of gas = 3.50 atm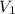 = initial volume of gas = 2.00 L
= initial volume of gas = 2.00 L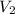 = final volume of gas = 20.0 L
= final volume of gas = 20.0 L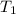 = initial temperature of gas =
= initial temperature of gas = 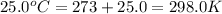
 = final temperature of gas = ?
= final temperature of gas = ?