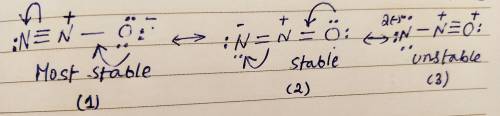Chemistry, 21.03.2020 03:16 winterblanco
Draw three resonance structures for N2O. This species has its three atoms bonded sequentially in the following fashion: N-O. Draw your resonance structures so that the atoms in them are bonded together in this order. Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. Now select the statement from the multiple choices which is true about this most important resonance structure. In the most important resonance structure of N2O :

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
Draw three resonance structures for N2O. This species has its three atoms bonded sequentially in the...
Questions

Physics, 08.02.2022 14:00


Biology, 08.02.2022 14:00



Chemistry, 08.02.2022 14:00


Mathematics, 08.02.2022 14:00



English, 08.02.2022 14:00

French, 08.02.2022 14:00



History, 08.02.2022 14:00


Chemistry, 08.02.2022 14:00