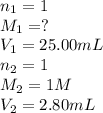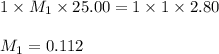Chemistry, 21.03.2020 03:13 ellisc7044
1. Determine the volume of 1M NaOH that is required to reach the equivalence point with 25.00 mL of HCl (of unknown concentration). From there, calculate the original concentration of the unknown HCl solution.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
1. Determine the volume of 1M NaOH that is required to reach the equivalence point with 25.00 mL of...
Questions



English, 27.02.2021 19:10

Mathematics, 27.02.2021 19:10


Mathematics, 27.02.2021 19:10

Mathematics, 27.02.2021 19:10



English, 27.02.2021 19:10


English, 27.02.2021 19:10

Mathematics, 27.02.2021 19:10



Mathematics, 27.02.2021 19:10

Mathematics, 27.02.2021 19:10



Social Studies, 27.02.2021 19:10


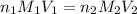
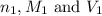 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 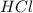
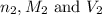 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.