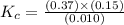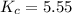Chemistry, 21.03.2020 02:58 alexisfaithsmith
At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus trichloride, the following concentrations are obtained: 0.010 mol/L PCl5, 0.15 mol/l PCl3 and 0.37 mol/L Cl2. Determine the Keq for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus...
Questions



Spanish, 13.01.2021 14:20

English, 13.01.2021 14:20


Mathematics, 13.01.2021 14:20


Physics, 13.01.2021 14:20



Social Studies, 13.01.2021 14:20

Physics, 13.01.2021 14:20

Mathematics, 13.01.2021 14:20


History, 13.01.2021 14:20


Mathematics, 13.01.2021 14:20

Mathematics, 13.01.2021 14:20


Mathematics, 13.01.2021 14:30

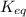 for the reaction is 5.55
for the reaction is 5.55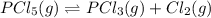
![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/0557/1142/ffe89.png)