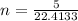Chemistry, 21.03.2020 02:57 sherlock19
Gas mixture consists of N2, O2, and Ne, where the mole fraction of N2 is 0.55 and the mole fraction of Ne is 0.25. If the mixture is at STP in a 5.0 L container, how many molecules of O2 are present?

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 12:50
Complete the paragraph to describe the characteristics of a borane molecule (bh3). the lewis structure and table of electronegativities are given. the bond polarities in bh3 are , the molecular shape is , and the molecule is .
Answers: 2

Chemistry, 23.06.2019 13:30
Where are electrons with the lowest energy found? in the nucleus farthest from the nucleus outside the atom closest to the nucleus
Answers: 1
You know the right answer?
Gas mixture consists of N2, O2, and Ne, where the mole fraction of N2 is 0.55 and the mole fraction...
Questions


Mathematics, 03.10.2019 05:30

History, 03.10.2019 05:30

Social Studies, 03.10.2019 05:30

Business, 03.10.2019 05:30

Computers and Technology, 03.10.2019 05:30

Computers and Technology, 03.10.2019 05:30



History, 03.10.2019 05:30


Biology, 03.10.2019 05:30

Mathematics, 03.10.2019 05:30

Mathematics, 03.10.2019 05:30



History, 03.10.2019 05:30


Chemistry, 03.10.2019 05:30