
Chemistry, 20.03.2020 22:41 sarahsompayrac
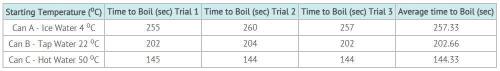
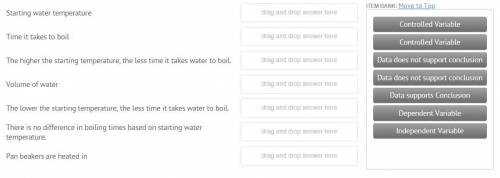
Sarah was studying phase change in science class. She believed that cold water would begin boiling sooner than warmer water. Sarah and his father did the following investigation. Hypothesis: The lower the water’s starting temperature, the quicker the water will start to boil. Experimental Design: Sarah and her dad used three different water temperatures. They measured 50 ml of water into 100 ml beakers. The temperature of the water was recorded. They placed the beakers in a large pan of water and placed the water on the burner of the stove. Sarah timed how long it took each container to boil. They also ran the experiment three times and took an average of their data. You can see the data in the table below.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Sarah was studying phase change in science class. She believed that cold water would begin boiling s...
Questions

French, 22.07.2021 19:40

Mathematics, 22.07.2021 19:40



Mathematics, 22.07.2021 19:40


History, 22.07.2021 19:40





Arts, 22.07.2021 19:40


Biology, 22.07.2021 19:40

Mathematics, 22.07.2021 19:40

Mathematics, 22.07.2021 19:40


Mathematics, 22.07.2021 19:40




