
Pure copper may be produced by the reaction of copper(I) sulfide with oxygen gas as follows: Cu2S(s) + O2(g) 2Cu(s) + SO2(g) If 0.680 kg of copper(I) sulfide reacts with excess oxygen, what mass of copper metal may be produced ? A) 0.680 kg B) 0.136 kg C) 0.271 kg D) 0.543 kg E) 1.36 kg

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1


Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3

Chemistry, 23.06.2019 13:00
Sort these isotopes by whether they are most likely to undergo fusion or fission. hydrogen-3, uranium-233, plutonium-239, hydrogen-1, helium-3, plutonium-241
Answers: 2
You know the right answer?
Pure copper may be produced by the reaction of copper(I) sulfide with oxygen gas as follows: Cu2S(s)...
Questions

History, 05.11.2020 18:50




Business, 05.11.2020 18:50





English, 05.11.2020 18:50



World Languages, 05.11.2020 18:50

Mathematics, 05.11.2020 18:50

World Languages, 05.11.2020 18:50

History, 05.11.2020 18:50

Mathematics, 05.11.2020 18:50

Biology, 05.11.2020 18:50


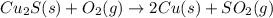
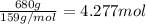
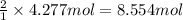 copper
copper

