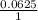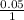Chemistry, 20.03.2020 12:03 j1theking18
When aqueous solutions of Na2SO4 and Pb(NO3)2 are mixed, PbSO4 precipitates. Calculate the mass of PbSO4 formed when 1.25 L of 0.0500 M Pb(NO3)2 and 2.00 L of 0.0250 M Na2SO4 are mixed.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
When aqueous solutions of Na2SO4 and Pb(NO3)2 are mixed, PbSO4 precipitates. Calculate the mass of P...
Questions

Mathematics, 18.11.2020 07:00

History, 18.11.2020 07:00

English, 18.11.2020 07:00

Mathematics, 18.11.2020 07:00

Mathematics, 18.11.2020 07:00

Mathematics, 18.11.2020 07:00


Biology, 18.11.2020 07:00


English, 18.11.2020 07:00

Mathematics, 18.11.2020 07:00

Mathematics, 18.11.2020 07:00




Biology, 18.11.2020 07:00




Mathematics, 18.11.2020 07:00