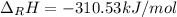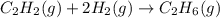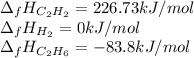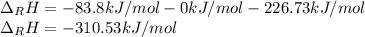Chemistry, 20.03.2020 12:02 tae8002001
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ/mole) the standard enthalpy change ΔH° for the hydrogenation of ethyne (acetylene) to ethane. Just enter a number (no units).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The wilson chamber is used to study: direction, speed, and distance of radioactivity the intensity of radiation the appearance of individual atoms all of the above
Answers: 1


Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1
You know the right answer?
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ/mole)...
Questions

Mathematics, 08.11.2020 14:00

English, 08.11.2020 14:00



Mathematics, 08.11.2020 14:00


Biology, 08.11.2020 14:00

Social Studies, 08.11.2020 14:00

Biology, 08.11.2020 14:00


Computers and Technology, 08.11.2020 14:00

Chemistry, 08.11.2020 14:00

Computers and Technology, 08.11.2020 14:00


Mathematics, 08.11.2020 14:00

English, 08.11.2020 14:00

Chemistry, 08.11.2020 14:00

Chemistry, 08.11.2020 14:00

Mathematics, 08.11.2020 14:00

English, 08.11.2020 14:00