
Chemistry, 20.03.2020 11:46 bnation5970
Iodine is prepared both in the laboratory and commercially by adding Cl 2 ( g ) to an aqueous solution containing sodium iodide. 2 NaI ( aq ) + Cl 2 ( g ) ⟶ I 2 ( s ) + 2 NaCl ( aq ) How many grams of sodium iodide, NaI , must be used to produce 86.1 g of iodine, I 2 ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1


Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Iodine is prepared both in the laboratory and commercially by adding Cl 2 ( g ) to an aqueous soluti...
Questions

Advanced Placement (AP), 25.06.2019 22:00









History, 25.06.2019 22:00


Mathematics, 25.06.2019 22:00

Mathematics, 25.06.2019 22:00



English, 25.06.2019 22:00

Mathematics, 25.06.2019 22:00



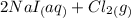 ⇒
⇒ 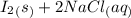
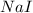 is required for the preparation of one mole of
is required for the preparation of one mole of 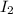 . In terms of mass we can say that, 299.78 grams of
. In terms of mass we can say that, 299.78 grams of 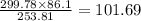 grams
grams

