
Chemistry, 20.03.2020 10:46 jdisalle476
A sample of an Iron Oxalato complex salt weighting 0.13 grams requires 32.74 mL of 0.01 M KMnO4 to turn the solution a very light pink color at the quivalence point. Calculate the number of moles of KMnO4 added.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
A sample of an Iron Oxalato complex salt weighting 0.13 grams requires 32.74 mL of 0.01 M KMnO4 to t...
Questions

English, 27.05.2020 11:58

Mathematics, 27.05.2020 11:58



Mathematics, 27.05.2020 11:58

Mathematics, 27.05.2020 11:58

Mathematics, 27.05.2020 11:58

Mathematics, 27.05.2020 11:58

Biology, 27.05.2020 11:58


Engineering, 27.05.2020 11:58



Biology, 27.05.2020 11:58





Mathematics, 27.05.2020 11:58

 added are 0.0003
added are 0.0003 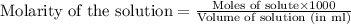 .....(1)
.....(1)


