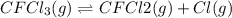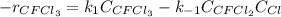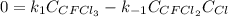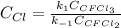Chemistry, 20.03.2020 10:54 diwashkandel6pe02af
Consider the following elementary reaction: CFC13(g)-CFC12(9)+Clg) Suppose we let k1 stand for the rate constant of this reaction, and k1 stand for the rate constant of the reverse reaction Write an expression that gives the equilibrium concentration of Cl in terms of k, k_1, and the equilibrium concentrations of CFCI3 and CFCI2 1. K-1 [ci]

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
Consider the following elementary reaction: CFC13(g)-CFC12(9)+Clg) Suppose we let k1 stand for the r...
Questions

Mathematics, 08.12.2021 23:40


Business, 08.12.2021 23:40

Social Studies, 08.12.2021 23:40



Mathematics, 08.12.2021 23:40


Mathematics, 08.12.2021 23:40

Social Studies, 08.12.2021 23:40

Computers and Technology, 08.12.2021 23:40

Mathematics, 08.12.2021 23:40

Mathematics, 08.12.2021 23:40

SAT, 08.12.2021 23:40


Mathematics, 08.12.2021 23:40

History, 08.12.2021 23:40


Health, 08.12.2021 23:40

Social Studies, 08.12.2021 23:40

![[Cl]_{eq}=\frac{k_1[CFCl_3]_{eq}}{k_{-1}[CFCl_2]_{eq}}](/tpl/images/0555/9861/640b9.png)