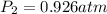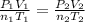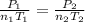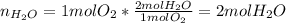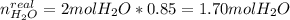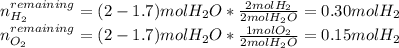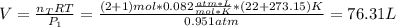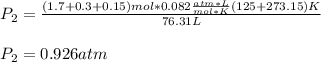A mixture in which the mole ratio of hydrogen to oxygen is (exactly) 2:1 is used to prepare water by the reaction 2 H2 (g) + O2 (g) → 2 H2O (g) The total pressure in the container is 0.951 atm at 22°C before the reaction. What is the final pressure in the container after the reaction, with a final temperature of 125°C, no volume change, and an 85.0% yield?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1


Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3
You know the right answer?
A mixture in which the mole ratio of hydrogen to oxygen is (exactly) 2:1 is used to prepare water by...
Questions

Mathematics, 05.11.2020 21:00

Spanish, 05.11.2020 21:00

Mathematics, 05.11.2020 21:00

Health, 05.11.2020 21:00

Social Studies, 05.11.2020 21:00


Mathematics, 05.11.2020 21:00






Mathematics, 05.11.2020 21:00

Geography, 05.11.2020 21:00



Biology, 05.11.2020 21:00

Social Studies, 05.11.2020 21:00


Mathematics, 05.11.2020 21:00