
Chemistry, 20.03.2020 10:06 masteroftheuniverse3
Solving for the pH in a mixture of acids is like dealing with a diprotic acid. You solve the problem by dealing with the acids in successive order. You start with the stronger acid (just like you start with the Ka1 because it is higher than the Ka2 for a diprotic). Afterward, you move on to the weaker acid. If a solution contains 0.029 M HCl and 0.100 M HF (Ka=6.6x10-4), what will be the pH of the solution?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
Solving for the pH in a mixture of acids is like dealing with a diprotic acid. You solve the problem...
Questions

Mathematics, 14.07.2019 09:00


Arts, 14.07.2019 09:00


SAT, 14.07.2019 09:00



Biology, 14.07.2019 09:00

Mathematics, 14.07.2019 09:00



Social Studies, 14.07.2019 09:00






Biology, 14.07.2019 09:00

Mathematics, 14.07.2019 09:00

![[H^+]](/tpl/images/0555/8561/07acb.png)
![[HCl]=[H^+]=0.029 M](/tpl/images/0555/8561/09dd5.png)
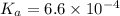
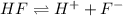
![K_a=\frac{[H^+][F^-]}{[HF]}=\frac{x\times x}{(c-x)}](/tpl/images/0555/8561/1ea44.png)
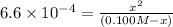
![[H^+]'=x=0.0078 M](/tpl/images/0555/8561/68e8c.png)
![[H^+]_t=[H^+]+[H^+]'=0.029 M + 0.0078 M=0.0368 M](/tpl/images/0555/8561/81922.png)
![pH=-\log[H^+]_t](/tpl/images/0555/8561/ac0ab.png)
![=-\log[0.0368 M]=1.43](/tpl/images/0555/8561/d2fa1.png)


