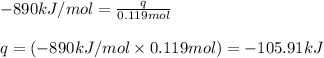Chemistry, 20.03.2020 09:52 KillerSteamcar
In the presence of excess oxygen, methane gas burns in a constant-pressure system Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. to yield carbon dioxide and water: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ΔH = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure. Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1


Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
In the presence of excess oxygen, methane gas burns in a constant-pressure system Calculate the valu...
Questions

Mathematics, 16.07.2019 15:00


Social Studies, 16.07.2019 15:00



Mathematics, 16.07.2019 15:00

Mathematics, 16.07.2019 15:00


Mathematics, 16.07.2019 15:00




History, 16.07.2019 15:00

Mathematics, 16.07.2019 15:00


Mathematics, 16.07.2019 15:00

English, 16.07.2019 15:00

Mathematics, 16.07.2019 15:00


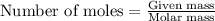
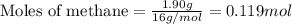
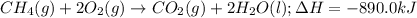
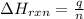
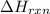 = enthalpy change of the reaction = -890.0 kJ/mol
= enthalpy change of the reaction = -890.0 kJ/mol