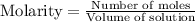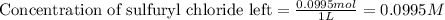Chemistry, 20.03.2020 09:55 solobiancaa
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g) follows first-order kinetics. At 320◦C the rate constant is 2.2 × 10−5 sec−1 . If one started with a sample containing 0.16 moles of sulfuryl chloride per liter at 320◦C, what concentration would be left after 6.00 hours?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g)...
Questions






Mathematics, 02.10.2019 06:10








English, 02.10.2019 06:10


Mathematics, 02.10.2019 06:10

History, 02.10.2019 06:10

Social Studies, 02.10.2019 06:10


Mathematics, 02.10.2019 06:10

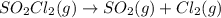
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0555/8016/f1041.png)
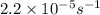
![[A_o]](/tpl/images/0555/8016/dc622.png) = initial amount of the sample = 0.16 moles
= initial amount of the sample = 0.16 moles![2.2\times 10^{-5}=\frac{2.303}{21600}\log\frac{0.16}{[A]}](/tpl/images/0555/8016/ba4a8.png)
![[A]=0.0995moles](/tpl/images/0555/8016/152d1.png)