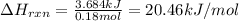Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
Answers: 1

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
2. When 15.3 g of sodium nitrate, NaNO3,was dissolved in water in a calorimeter, the temperature fel...
Questions

Mathematics, 01.12.2020 20:00


English, 01.12.2020 20:00




Mathematics, 01.12.2020 20:00



Social Studies, 01.12.2020 20:00


Business, 01.12.2020 20:00

Mathematics, 01.12.2020 20:00





History, 01.12.2020 20:00


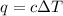
 = change in temperature =
= change in temperature = 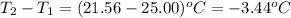
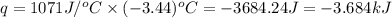
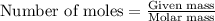
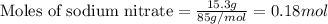
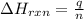
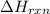 = enthalpy change of the reaction
= enthalpy change of the reaction