
Chemistry, 20.03.2020 09:57 nommies005
4. A solution of the weak base, ammonia (NH3), was completely neutralized with the strong acid HCl. Write out the dominant equilibrium (including phase labels) that would exist in this "neutralized" solution. HINT: first look at what ions would exist in solution after the non-equilibrium reaction with the strong acid is over. Second, decide if either of these ions is an acid or a base. Lastly, write the equation for this ion acting as an acid or base in water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
4. A solution of the weak base, ammonia (NH3), was completely neutralized with the strong acid HCl....
Questions

Mathematics, 09.03.2021 22:40

Mathematics, 09.03.2021 22:40


Mathematics, 09.03.2021 22:40







Physics, 09.03.2021 22:40

Chemistry, 09.03.2021 22:40


History, 09.03.2021 22:40

English, 09.03.2021 22:40

Mathematics, 09.03.2021 22:40

Chemistry, 09.03.2021 22:40

English, 09.03.2021 22:40


English, 09.03.2021 22:40

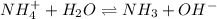
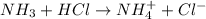
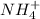 and
and 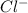 ions exist.
ions exist.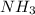 is an weak base and HCl is a strong acid.
is an weak base and HCl is a strong acid.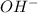 .
.


