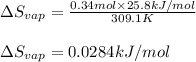Chemistry, 20.03.2020 07:24 ramanpreet
The molar heat of vaporization of pentane is 25.8 kJ·mol−1, and the boiling point of pentane is 36.1°C. Calculate the value of ΔvapS for the vaporization of 0.34 mole of pentane.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

You know the right answer?
The molar heat of vaporization of pentane is 25.8 kJ·mol−1, and the boiling point of pentane is 36.1...
Questions


Mathematics, 02.09.2021 21:40




History, 02.09.2021 21:40


Physics, 02.09.2021 21:40



Mathematics, 02.09.2021 21:40



Computers and Technology, 02.09.2021 21:40





Mathematics, 02.09.2021 21:40

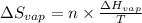
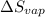 = Entropy change of vaporization = ?
= Entropy change of vaporization = ?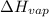 = molar heat of vaporization = 25.8 kJ/mol
= molar heat of vaporization = 25.8 kJ/mol![36.1^oC=[36.1+273]K=309.1K](/tpl/images/0555/6193/0c07c.png)