Chemistry, 20.03.2020 05:50 jonystroyer1020
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an enthalpy of 498 kJ/mol , and the standard enthalpy of formation of ClO2 102.5 kJ/mol , calculate the value for the enthalpy of formation per mole of ClO(g). What is the value for the enthalpy of formation per mole of ClO(g)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an entha...
Questions





Mathematics, 11.01.2022 09:40




Chemistry, 11.01.2022 09:40


Mathematics, 11.01.2022 09:50

Social Studies, 11.01.2022 09:50

Geography, 11.01.2022 09:50


Mathematics, 11.01.2022 09:50




English, 11.01.2022 09:50

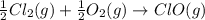
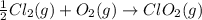 ;
; 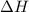 = 102.5 kJ
= 102.5 kJ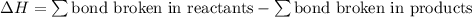
![[(\frac{1}{2})x + 498] - [(2)(243)]](/tpl/images/0555/5141/c814b.png)
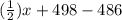
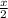
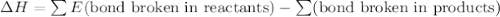
![[(\frac{1}{2})181 + (\frac{1}{2})498] - 243](/tpl/images/0555/5141/97422.png)