
Calculate the density of oxygen, O2, under each of the following conditions: STP 1.00 atm and 35.0 ∘C
Express your answers numerically in grams per liter. Enter the density at STP first and separate your answers by a comma. density at STP, density at 1 atm and 35.0 ∘C= g/L

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Calculate the density of oxygen, O2, under each of the following conditions: STP 1.00 atm and 35.0 ∘...
Questions


English, 13.11.2019 17:31

History, 13.11.2019 17:31

Mathematics, 13.11.2019 17:31

History, 13.11.2019 17:31





Mathematics, 13.11.2019 17:31

Physics, 13.11.2019 17:31



Chemistry, 13.11.2019 17:31


Health, 13.11.2019 17:31

Physics, 13.11.2019 17:31


Health, 13.11.2019 17:31

Computers and Technology, 13.11.2019 17:31

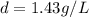 at STP,
at STP,  at 1 atm and
at 1 atm and 
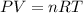
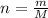

 which is known as density of the gas
which is known as density of the gas

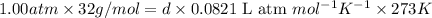
 :
: 



