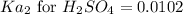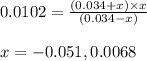Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
Suppose a 0.034 M aqueous solution of sulfuric acid (H2SO4) is prepared. Calculate the equilibrium m...
Questions

Mathematics, 09.03.2021 17:50

History, 09.03.2021 17:50


Mathematics, 09.03.2021 17:50


Business, 09.03.2021 17:50


Mathematics, 09.03.2021 17:50

History, 09.03.2021 17:50

Mathematics, 09.03.2021 17:50



Mathematics, 09.03.2021 17:50

Mathematics, 09.03.2021 17:50


Mathematics, 09.03.2021 17:50

Mathematics, 09.03.2021 17:50

Mathematics, 09.03.2021 17:50


Mathematics, 09.03.2021 17:50

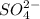 at equilibrium is 0.0068 M
at equilibrium is 0.0068 M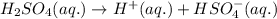
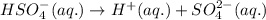
![Ka_2=\frac{[H^+][SO_4^{2-}]}{[HSO_4^-]}](/tpl/images/0555/4892/83003.png)