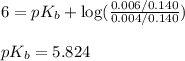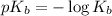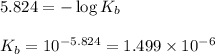Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1
You know the right answer?
A student dissolves 0.0100 mole of an unknown weak base in 100.00 mL water and titrates the
s...
s...
Questions



History, 01.10.2019 10:20

Physics, 01.10.2019 10:20



Social Studies, 01.10.2019 10:30

History, 01.10.2019 10:30

History, 01.10.2019 10:30


Computers and Technology, 01.10.2019 10:30


English, 01.10.2019 10:30


Spanish, 01.10.2019 10:30




Business, 01.10.2019 10:30

Social Studies, 01.10.2019 10:30

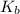 for weak base is
for weak base is 
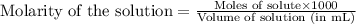
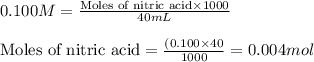
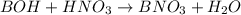
![pOH=pK_a+\log(\frac{[salt]}{[base]})](/tpl/images/0555/4585/13872.png)
![pOH=pK_b+\log(\frac{[BNO_3]}{[BOH]})](/tpl/images/0555/4585/b566f.png)
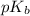 = negative logarithm of acid dissociation constant of formic acid = ?
= negative logarithm of acid dissociation constant of formic acid = ?
![[BNO_3]=\frac{0.004}{0.140}](/tpl/images/0555/4585/7cd20.png)
![[BOH]=\frac{0.006}{0.140} ](/tpl/images/0555/4585/f5b65.png)