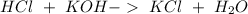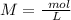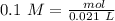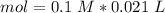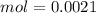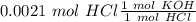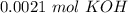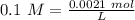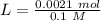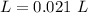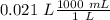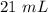Chemistry, 20.03.2020 03:34 emileehogan
Two 21.0 mL samples, one 0.100 M HCl and the other 0.100 M HF, were titrated with 0.200 M KOH. Answer each of the following questions regarding these two titrations. You may want to reference (Pages 755 - 769) Section 17.4 while completing this problem. Part A What is the volume of added base at the equivalence point for HCl

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

You know the right answer?
Two 21.0 mL samples, one 0.100 M HCl and the other 0.100 M HF, were titrated with 0.200 M KOH. Answe...
Questions

Mathematics, 15.06.2021 21:50





Mathematics, 15.06.2021 21:50

Mathematics, 15.06.2021 21:50

Mathematics, 15.06.2021 21:50


Biology, 15.06.2021 21:50

History, 15.06.2021 21:50



Business, 15.06.2021 21:50





Mathematics, 15.06.2021 21:50