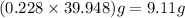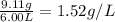Chemistry, 20.03.2020 03:02 twinchristiansp4xhd2
A 0.228 mol sample of Ar gas is contained in a 6.00 L flask at room temperature and pressure. What is the density of the gas, in grams/liter, under these conditions?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1


Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
A 0.228 mol sample of Ar gas is contained in a 6.00 L flask at room temperature and pressure. What i...
Questions

English, 27.02.2020 23:26

Mathematics, 27.02.2020 23:26

History, 27.02.2020 23:26

Health, 27.02.2020 23:26


Chemistry, 27.02.2020 23:26










Biology, 27.02.2020 23:26




History, 27.02.2020 23:27