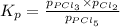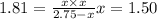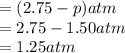At 250°C the equilibrium constant (Kp) for the reaction below is 1.81. PCl5(g) PCl3(g) + Cl2(g) Sufficient PCl5 is put into a vessel to give an initial pressure of 2.75 atm at 250°C. What will be the final pressure at this temperature after the system has reached equilibrium?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
At 250°C the equilibrium constant (Kp) for the reaction below is 1.81. PCl5(g) PCl3(g) + Cl2(g) Suff...
Questions





Mathematics, 19.05.2020 10:58




Mathematics, 19.05.2020 10:58



Mathematics, 19.05.2020 10:58

Mathematics, 19.05.2020 10:58

Mathematics, 19.05.2020 10:58

Mathematics, 19.05.2020 10:58




Mathematics, 19.05.2020 10:58

Health, 19.05.2020 10:58

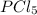 at equilibrium is 1.25 atm
at equilibrium is 1.25 atm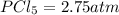
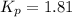
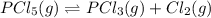
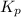 for the given reaction
for the given reaction