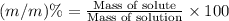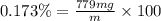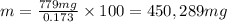Chemistry, 20.03.2020 02:35 StephenCurry34
You can practice converting between the mass of a solution and mass of solute when the mass percent concentration of a solution is known. The concentration of the KCN solution given in Part A corresponds to a mass percent of 0.173 %. What mass of a 0.173 % KCN solution contains 779 mg of KCN

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1

Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
You can practice converting between the mass of a solution and mass of solute when the mass percent...
Questions

Mathematics, 27.07.2019 10:30



Advanced Placement (AP), 27.07.2019 10:30

Physics, 27.07.2019 10:30




English, 27.07.2019 10:30






Geography, 27.07.2019 10:30





Biology, 27.07.2019 10:30