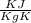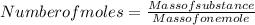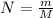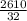Chemistry, 20.03.2020 01:55 normarjohnson
An "empty" container is not really empty if it contains air. How may moles of oxygen are in an "empty" two-liter cola bottle at atmospheric pressure (1 atm) and room temperature (25∘C)? Assume ideal behavior.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
An "empty" container is not really empty if it contains air. How may moles of oxygen are in an "empt...
Questions

Mathematics, 07.02.2021 01:00


English, 07.02.2021 01:00

Mathematics, 07.02.2021 01:00



Mathematics, 07.02.2021 01:00

Mathematics, 07.02.2021 01:00


English, 07.02.2021 01:00

History, 07.02.2021 01:00

History, 07.02.2021 01:00

History, 07.02.2021 01:00

English, 07.02.2021 01:00



History, 07.02.2021 01:00

Business, 07.02.2021 01:00


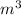
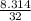 = 0.26
= 0.26