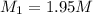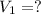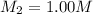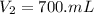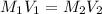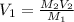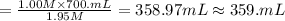A chemist must prepare 700.mL of 1.00M aqueous calcium bromide CaBr2 working solution. He'll do this by pouring out some 1.95M aqueous calcium bromide stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the calcium bromide stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
A chemist must prepare 700.mL of 1.00M aqueous calcium bromide CaBr2 working solution. He'll do this...
Questions

History, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00




Mathematics, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00


History, 12.12.2020 16:00



Advanced Placement (AP), 12.12.2020 16:00

Mathematics, 12.12.2020 16:00



Social Studies, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00