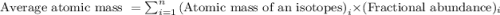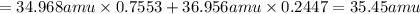Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1


Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 06:20
What is the magnitude of the force of gravity between to 1000 kg cars which are separated by distance of 25. 0 km on an interstate highway? the force between the two cars will be what
Answers: 3
You know the right answer?
The atomic masses of 35-Cl (75.53 percent) and 37-Cl (24.47 percent) are 34.968 amu and 36.956 amu,...
Questions

Mathematics, 23.10.2019 21:00



Computers and Technology, 23.10.2019 21:00


Chemistry, 23.10.2019 21:00




Mathematics, 23.10.2019 21:00




Health, 23.10.2019 21:00

Computers and Technology, 23.10.2019 21:00

Mathematics, 23.10.2019 21:00


Mathematics, 23.10.2019 21:00