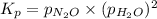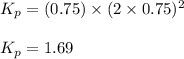Chemistry, 20.03.2020 00:42 Wolfgirl2032
A sample of solid NH4NO3 is placed in an empty container. It decomposes according to the following reaction: NH4NO3(s) LaTeX: \Leftrightarrow⇔ N2O(g) + 2H2O(g) At equilibrium, the total pressure in the container is 2.25 atm. Calculate Kp.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
A sample of solid NH4NO3 is placed in an empty container. It decomposes according to the following r...
Questions

Mathematics, 22.12.2020 22:40

Mathematics, 22.12.2020 22:40

Computers and Technology, 22.12.2020 22:40

History, 22.12.2020 22:40

Mathematics, 22.12.2020 22:40

Mathematics, 22.12.2020 22:40

Social Studies, 22.12.2020 22:40






Mathematics, 22.12.2020 22:40



Mathematics, 22.12.2020 22:40

Mathematics, 22.12.2020 22:40


Mathematics, 22.12.2020 22:40

History, 22.12.2020 22:40

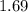
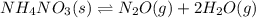
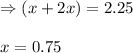
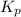 for above equation follows:
for above equation follows: