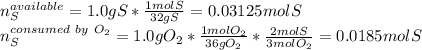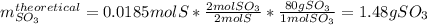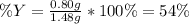Chemistry, 20.03.2020 00:28 madisonenglishp2qkow
Sulfur and oxygen react in a combination reaction to produce sulfur trioxide, an environmental pollutant: 2S(s) + 3O2(g) → 2SO3(g) In a particular experiment, the reaction of 1.0 g S with 1.0 g O2 produced 0.80 g of SO3. The % yield in this experiment is .

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
Sulfur and oxygen react in a combination reaction to produce sulfur trioxide, an environmental pollu...
Questions



Mathematics, 31.12.2020 21:30

Chemistry, 31.12.2020 21:30



Mathematics, 31.12.2020 21:40

Mathematics, 31.12.2020 21:40




Mathematics, 31.12.2020 21:40

Arts, 31.12.2020 21:40

Mathematics, 31.12.2020 21:40

Business, 31.12.2020 21:40