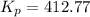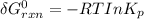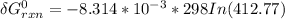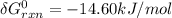Chemistry, 19.03.2020 23:59 Lesquirrel
Consider the equilibrium A(g) ⇀↽ 2 B(g) + 3 C(g) at 25◦C. When A is loaded into a cylinder at 9.13 atm and the system is allowed to come to equilibrium, the final pressure is found to be 16.89 atm. What is ∆G ◦ r for this reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

You know the right answer?
Consider the equilibrium A(g) ⇀↽ 2 B(g) + 3 C(g) at 25◦C. When A is loaded into a cylinder at 9.13 a...
Questions

English, 25.09.2021 09:30


Mathematics, 25.09.2021 09:30

Mathematics, 25.09.2021 09:30

Mathematics, 25.09.2021 09:30

Mathematics, 25.09.2021 09:30

Mathematics, 25.09.2021 09:30

Computers and Technology, 25.09.2021 09:30

Mathematics, 25.09.2021 09:30


History, 25.09.2021 09:30

Mathematics, 25.09.2021 09:30

Mathematics, 25.09.2021 09:30

Mathematics, 25.09.2021 09:30

Mathematics, 25.09.2021 09:30


History, 25.09.2021 09:30

Health, 25.09.2021 09:30


Advanced Placement (AP), 25.09.2021 09:30

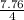
![K_p=\frac{[P_3]^2[P_c]^3}{[P_a]}](/tpl/images/0554/9307/a7e62.png)
![K_p=\frac{[3.88]^2[5.82]^3}{[7.19]}](/tpl/images/0554/9307/cde43.png)