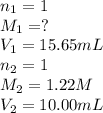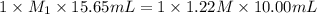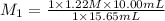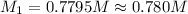Chemistry, 19.03.2020 23:59 michael1498
In a titration, 15.65 milliliters of a KOH * (aq) ) solution exactly neutralized 10.00 milliliters of a 1.22 M HCl * (aq) solution . In the space below , show a correct numerical setup for calculating the molarity of the KOH * (aq) solution .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1


You know the right answer?
In a titration, 15.65 milliliters of a KOH * (aq) ) solution exactly neutralized 10.00 milliliters o...
Questions




Biology, 27.07.2019 07:30


Mathematics, 27.07.2019 07:30

English, 27.07.2019 07:30


Mathematics, 27.07.2019 07:30

Mathematics, 27.07.2019 07:30

Mathematics, 27.07.2019 07:30

Chemistry, 27.07.2019 07:30

Computers and Technology, 27.07.2019 07:30


Geography, 27.07.2019 07:30




Mathematics, 27.07.2019 07:30

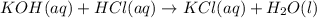
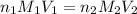
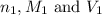 are the n-factor, molarity and volume of acid which is KOH
are the n-factor, molarity and volume of acid which is KOH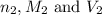 are the n-factor, molarity and volume of base which is HCl.
are the n-factor, molarity and volume of base which is HCl.