
Chemistry, 20.03.2020 00:06 buddyclayjohnson
A mixture of methane (CH4) and ethane (C2H6) is stored in a container at 294 mm Hg. The gases are burned in air to form CO2 and H2O. If the pressure of CO2 is 351 mm Hg measured at the same temperature and volume as the original mixture, calculate the mole fraction of the gases.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

You know the right answer?
A mixture of methane (CH4) and ethane (C2H6) is stored in a container at 294 mm Hg. The gases are bu...
Questions

History, 02.10.2019 07:30

Mathematics, 02.10.2019 07:30

Mathematics, 02.10.2019 07:30


Mathematics, 02.10.2019 07:30

Mathematics, 02.10.2019 07:30


Biology, 02.10.2019 07:30

Biology, 02.10.2019 07:30

Biology, 02.10.2019 07:30



Biology, 02.10.2019 07:30

History, 02.10.2019 07:30

Mathematics, 02.10.2019 07:30

Mathematics, 02.10.2019 07:30




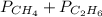 = 294 mm Hg
= 294 mm Hg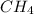 will yield 1 mole of
will yield 1 mole of 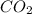 whereas 1 mole of
whereas 1 mole of 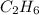 will yield 2 moles of
will yield 2 moles of 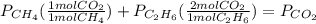
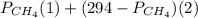 = 351 mm Hg
= 351 mm Hg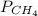 = 2(294) - 351
= 2(294) - 351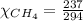
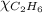 = 1 - 0.806
= 1 - 0.806


