
Chemistry, 19.03.2020 23:33 littledudefromacross
A sample of helium (He) gas initially at 19°C and 1.0 atm is expanded from 1.4 L to 2.8 L and simultaneously heated to 35°C. Calculate the entropy change for the process.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
A sample of helium (He) gas initially at 19°C and 1.0 atm is expanded from 1.4 L to 2.8 L and simult...
Questions














Mathematics, 16.07.2020 03:01






Mathematics, 16.07.2020 03:01

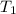 = (19 + 273) K = 292 K,
= (19 + 273) K = 292 K, 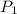 = 1.0 atm,
= 1.0 atm,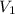 = 1.4 L
= 1.4 L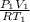
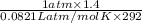
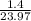
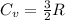
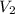 ) of 2.8 L and it is heated to
) of 2.8 L and it is heated to 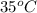 or (35 + 273) K = 308 K.
or (35 + 273) K = 308 K.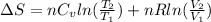
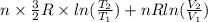
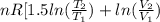
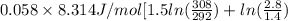
![0.4822 \times [1.5 \times 0.052 + 0.693]](/tpl/images/0554/8697/09f71.png)


