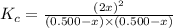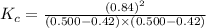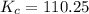Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
A sealed 1.0 L flask is charged with 0.500 mol of I2 and 0.500 mol of Br2. An equilibrium reaction e...
Questions

Social Studies, 06.04.2020 05:21

Mathematics, 06.04.2020 05:22


Chemistry, 06.04.2020 05:22

Mathematics, 06.04.2020 05:22

Mathematics, 06.04.2020 05:22

English, 06.04.2020 05:22

Mathematics, 06.04.2020 05:22


Health, 06.04.2020 05:23


History, 06.04.2020 05:23



Mathematics, 06.04.2020 05:24

Computers and Technology, 06.04.2020 05:24




Chemistry, 06.04.2020 05:24

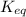 is 110.25
is 110.25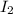 = 0.500 mole
= 0.500 mole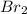 = 0.500 mole
= 0.500 mole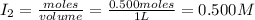
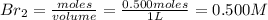
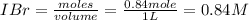 [/tex]
[/tex]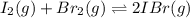
![K_c=\frac{[IBr]^2}{[Br_2]\times [I_2]}](/tpl/images/0554/7930/da4ed.png)