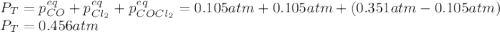CO(g) + Cl2 (g) ⇌ COCl2 (g) Kp = 22.5 at 395 °C . A sample of COCl2 (g) is introduced into a constantvolume vessel at 395 °C and observed to exert an initial pressure of 0.351 atm. When equilibrium is established, what will be the total pressure within the vessel?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1


Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2
You know the right answer?
CO(g) + Cl2 (g) ⇌ COCl2 (g) Kp = 22.5 at 395 °C . A sample of COCl2 (g) is introduced into a constan...
Questions

Computers and Technology, 03.03.2022 06:40



Mathematics, 03.03.2022 06:50




Mathematics, 03.03.2022 06:50


English, 03.03.2022 06:50


Mathematics, 03.03.2022 06:50


Computers and Technology, 03.03.2022 06:50




Computers and Technology, 03.03.2022 07:00


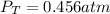
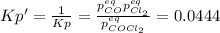
 due to reaction's progress, one obtains:
due to reaction's progress, one obtains: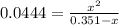
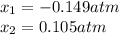
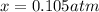 for which the total pressure at equilibrium is:
for which the total pressure at equilibrium is: