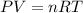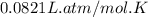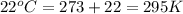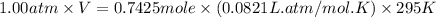Chemistry, 19.03.2020 21:25 xemnas1994
Automobile airbags contain solid sodium azide, NaN3,that reacts to produce nitrogen gas when heated, thus inflating the bag.2NaN3(s)⟶2Na(s)+3N2(g)Calculate the value of work, with, for the system if32.2NaNO3 reacts completely at1.00 atmand22∘C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2
You know the right answer?
Automobile airbags contain solid sodium azide, NaN3,that reacts to produce nitrogen gas when heated,...
Questions



Chemistry, 27.02.2021 15:40




Social Studies, 27.02.2021 15:40


Mathematics, 27.02.2021 15:40

English, 27.02.2021 15:40

Mathematics, 27.02.2021 15:40

Mathematics, 27.02.2021 15:40


Mathematics, 27.02.2021 15:40




Mathematics, 27.02.2021 15:40


Mathematics, 27.02.2021 15:40

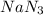
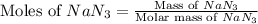
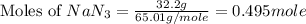
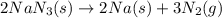
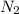
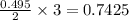 moles of
moles of